By Adam Gaffney, instructor in medicine 1, Joel Lexchin professor emeritus 2, US, Canadian Pharmaceutical Policy Reform Working Group
BMJ, Analysis, May 2018
To read the full proposal, see below or visit the BMJ website.
To access our economic analysis and supplemental materials, click here.
To read PNHP’s press release, click here.
To read and view media coverage of the proposal, click here.
Click on each section to see text.
Introduction
Drugs are among medicine’s most powerful tools. Yet the pharmaceutical systems of the United States and Canada are mired in dysfunction. The industry’s pricing practices—charging whatever the market will bear, especially in the US—strain budgets and put vital medicines out of reach for many patients.1-4 Despite some notable advances, the industry’s overall rate of real innovation remains incommensurate with our vast drug spending; many new drugs are marketed each year but few represent substantial clinical improvements.5-7 And commercial imperatives distort drug trials,8 research priorities, and drug regulation.9,10
While many recognize the need for change, proposed remedies vary3,11-13 and would fall short of achieving the fundamental reform that these deficiencies call for. The advocacy organization Physicians for a National Health Program therefore encouraged a working group of US and Canadian doctors, scholars, and advocates (the US/Canadian Pharmaceutical Policy Reform Working Group) to come together to craft a wide ranging reform proposal for both nations. Although political circumstances, including the influence of the pharmaceutical lobby, make full implementation of these reforms unlikely at present, shifting political winds may bring a more favorable policy climate. Hence, the working group aimed to craft an ambitious proposal for pharmaceutical reform to set an agenda for the future, including insurance coverage, pricing, drug development, clinical testing, regulatory approval, postmarketing monitoring, and promotion.
Although some of our recommendations (box 1) could be implemented within the existing US healthcare financing framework, full implementation would require a universal single payer system. Canada already has a single payer system but it would still require reforms because the system fully covers hospital and doctors’ services but not drugs out of hospital.14,15
Our proposal rests on six principles:
- Medical needs, not financial means, should determine access to medications
- Drugs must be affordable to society
- Drug development should be geared toward real innovation that maximizes population health
- The human right to health16 must take precedence over intellectual property rights (patents)
- The safety and effectiveness of medications must be independently and rigorously evaluated
- Comprehensive and unbiased information on drugs should be available to prescribers and patients.17
Summary of proposed pharmaceutical reforms
Access to prescription drugs
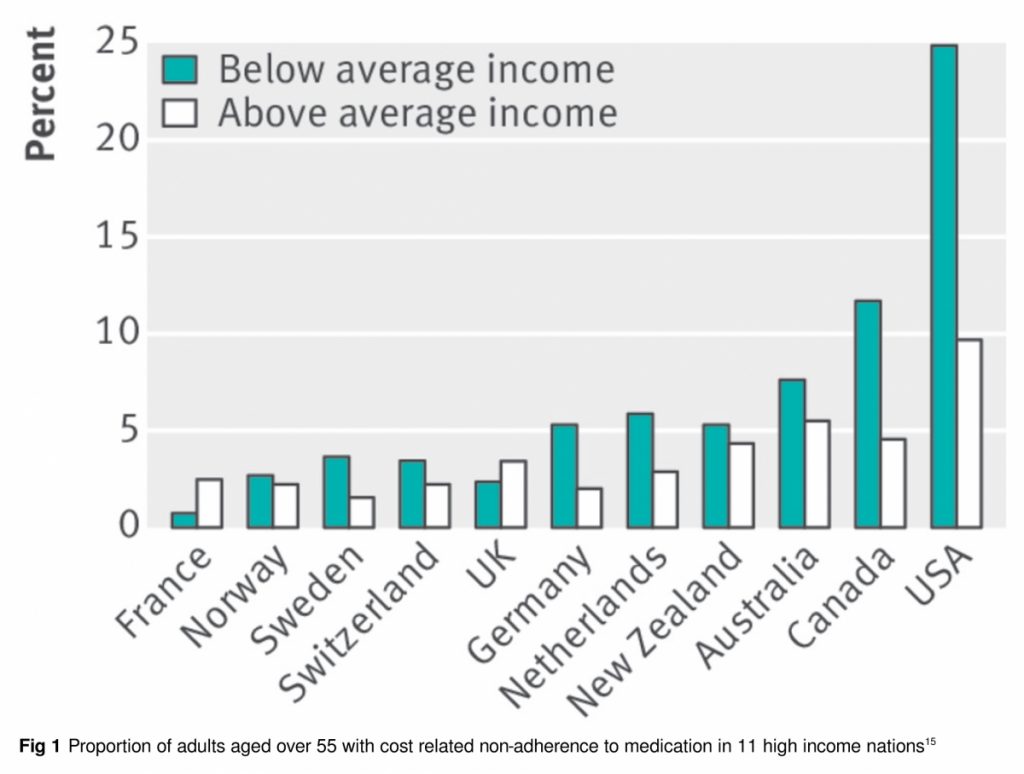
The right to essential medications is often compromised in both the US and Canada (fig 1). High out-of-pocket costs leave millions unable to fill prescriptions14,15,18 and drive many into bankruptcy.19,20 In the US an estimated 28 million people remain uninsured for healthcare,21 while 3.5 million in Canada lack drug coverage.14
Cost sharing (copayments, deductibles, and co-insurance) also impedes access on both sides of the border.15,22 It reduces needed and unnecessary care to similar degrees23; is a factor in reduced adherence24,25; and, for some conditions, exacerbates racial disparities in health,26 raises non-drug healthcare spending, and worsens outcomes.24,26 Notably, Wales, Northern Ireland, and Scotland have been able to provide universal drug coverage without cost sharing while using other cost control mechanisms to hold drug spending well below US or Canadian levels.27,28
To improve access and population health, we propose universal,29 first dollar coverage (full insurance with no cost sharing) of all medically necessary drugs, echoing Archie Cochrane’s famous invocation that “all effective treatments must be free.”30 Each nation should establish a national formulary of covered drugs, which should include all medications shown to improve the length or quality of life—or the safest, most effective, and least expensive option when equivalent agents are available. A national technology assessment office would provide data on comparative effectiveness to guide formulary decisions. When clinically appropriate—eg, for allergies or other unique circumstances—off-formulary drugs should also be covered.
Drug prices
Spending on outpatient drugs is higher in the US ($1026 (£742; €833) per capita annually) and Canada ($713) than in other nations in the Organization for Economic Cooperation and Development (averaging $515, and as low as $240 in Denmark).27 High prices (especially in the US) rather than high use explain these differences. For example, in 2014 a daily 50 unit dose of insulin glargine cost $186.38 a month in the US (after applicable discounts) versus $63.65 in the UK and $46.60 in France.31
Despite claims to the contrary, research and development costs cannot justify these high prices.32 For instance, the total research and development expenditures of 10 firms that recently introduced new cancer drugs amounted to $9bn, while those drugs generated $67bn in revenues.33 Drug firms continue to sharply increase US prices decades after recouping development costs,1,34-36 and their mean profits are consistently threefold higher than the average of other Fortune 500 firms—23% v 7% in 2016.37
Several steps could reduce drug prices while ensuring that no uniquely effective medications are withheld. Each nation’s regulatory agency would continue to approve drugs without regard to price. Once approved, however, a public agency would negotiate with manufacturers over prices, guided (in part) by comparative effectiveness data. Experience internationally, and in the US, indicates that such negotiations can lower prices38—probably by about 50% for branded drugs in the US.27,39-41
While negotiations and a national formulary could reduce prices for many medications, when patented drugs lack competitors firms could still demand unreasonable prices, forcing nations to exclude the drug or strain their budgets.42,43 Hence, additional options to assure reasonable pricing are necessary (fig 2). For instance, if price negotiations over branded drugs failed, governments would issue a compulsory license to allow generic manufacturing, a mechanism already sanctioned under international trade law,44 US patent law,45 and the Bayh-Dole Act.46 Indeed, in 2001, both the Bush (US)44,47 and Chretien (Canada) administrations,48 facing fears of anthrax bioterrorism, threatened to break the patent on ciprofloxacin, causing Bayer to lower the price.
In some circumstances, however, even compulsory licensing might not give reasonable prices; the cost of some generic drugs has soared after sole generic manufacturers cornered the market.1,35 We thus advocate creating public manufacturing capacity to produce drugs when no reasonably priced option is available. This capacity could also augment production during public health emergencies or drug shortages.49
Finally, drugs developed through public funding by public entities would remain unpatented and available for generic manufacture worldwide at greatly reduced cost.
Preclinical drug development
The patent protection and market exclusivity that prop up drug prices are typically portrayed as critical to encourage innovation. This portrayal is misleading for two reasons.
Firstly, despite achieving some important advances, the drug industry’s record on innovation is derisory relative to its vast revenues and profits.50 Most new drugs offer little new besides higher cost,2,6,7,51-55 while firms often extend market exclusivity through trivial modifications and secondary patenting.56,57
Secondly, it is far from clear that patents are the most important stimulus to therapeutic advance. Throughout history, curiosity and the intrinsic rewards of discovery, rather than financial incentives, have often driven scientific breakthroughs. Even today, most basic research underlying later drug innovation is carried out in non-profit or public institutions and funded by the National Institutes of Health (NIH) and the Canadian Institutes of Health Research (CIHR). Before the 1980 Bayh-Dole Act, the fruits of publicly funded research remained in the public domain in the US. Since 1980, however, publicly funded researchers have been allowed to patent their discoveries and sell them to drug firms,58 as occurred with the hepatitis C drug sofosbuvir. Although Bayh-Dole permits government to break the patents of such drugs, this provision has never been used.46
Thus, we propose the repeal of Bayh-Dole to keep drugs developed with public funding in the public domain. Meanwhile, for drugs developed fully by the private sector, the patent system should be reformed to encourage innovative drugs, not more look alike, “me-too” agents.
In the US, the criteria for issuing drug patents have been stretched far beyond the original requirement that a patentable discovery had to be useful, novel, and non-obvious.57,59 As others have argued,3,60,61 patent reforms could both lower prices and advance innovation. Minor variations or combinations of existing agents, drug isomers,3 and tweaks to drug delivery devices that don’t add important functionality should not be patentable. Some countries have already mandated similar restrictions.62
Because the reforms we advocate risk reducing incentives for industry to develop marketable products from important new discoveries, we propose creating institutes for prescription drug development within the NIH and CIHR. The new institutes would have two divisions: for drug innovation and for clinical trials (fig 2). The drug innovation division would focus on the development of non-patentable agents to the point of clinical trials. This “public track” would—alongside private research—fund the development of novel pharmaceuticals. We propose public funding equal to about half of current preclinical private sector investment. All novel molecules developed by the division would remain in the public domain. This approach is a form of “delinkage” of drug development and pricing that others have proposed.63,64
The drug innovation divisions might do some drug development themselves but would mostly fund efforts by academic or other non-commercial investigators. Priority would be given to potential drugs with the most clinical value, focusing on diseases that are neglected, commercially unprofitable, lacking effective treatments, or important for public health. The new, unpatented agents could be produced as generics by companies anywhere—a major advance for global health.
Clinical testing
Industry sponsored clinical trials have sometimes used unsound methods and reported incomplete findings, calling into question the interpretability, and sometimes the veracity, of their conclusions on safety and efficacy.8 For instance, trials have compared new agents with placebos rather than the best existing therapies, underdosed comparator drugs, or relied on surrogate endpoints65 that may not predict outcomes. Some commercially funded researchers have also selectively published (and republished) positive results8,66 or concealed negative findings,67 while firms have ended trials prematurely for purely commercial reasons.68
Meanwhile, corporate ownership of trial data can obscure safety problems and impede further research.69 Although requiring preregistration of trials has been an important step forward, transparency problems persist.70
Drug regulatory agencies must therefore raise evidentiary standards. Trials should, whenever possible, compare new agents to existing therapies and use a superiority design to discourage investment in unneeded me-too drugs. When new agents mimic existing ones, they should generally be tested in patients who do not respond to (or tolerate) existing products. And with infrequent exceptions, trials should assess hard clinical (rather than surrogate) outcomes.71 Anonymized patient level data from all trials (including older trials), should be made publicly available70 (whether or not a drug gains approval) to facilitate accountability and further research.
Finally, because of concerns regarding the objectivity of industry funded trials and the need to test unpatented and unprofitable therapies, the clinical trials divisions within the new NIH and CIHR institutes would also fund and oversee trials (fig 2).69,72 The divisions would select promising molecules developed by non-profit laboratories, academic investigators, and drug companies for clinical trials, which would mainly be designed and conducted by non-commercial investigators. They might also fund trials assessing new indications for existing agents or non-drug therapies.
Publicly funded trials would offer important benefits: minimizing commercial conflicts of interest; redirecting research from “me-too” drugs toward real innovations, and facilitating the development of unprofitable but essential treatments.69,72 Although firms could still fund trials of their products,72 because clinical trials are costly and would be subject to enhanced regulatory scrutiny (based on past evidence of companies manipulating results), publicly funded trials would be likely to predominate in the long term.
Drug approval reform
Canadian and US regulatory agencies too often allow unsafe drugs to reach the market73-76 and inadequately monitor them after approval.77-79 Both agencies’ independence has been eroded by their reliance, starting in the 1990s, on funding from fees paid by drug companies. In the US, the FDA’s receipt of these funds is explicitly linked to its shortening of review times.73,75
Meanwhile, an increasing proportion of new drugs qualify for programs that further reduce review times. By 2014, 69% of drugs submitted to the FDA gained “expedited review” through various designations or pathways.80 The comparable figure for Canada for 1997-2012 was 26%.81 Although intended to accelerate the availability of innovative agents, these programs have been exploited to speed the marketing of many “me-too” drugs.7,80 Some of these expedited review pathways have weaker standards of evidence. The recently enacted 21st Century Cures Act in the US creates even more such pathways, and mandates that the FDA evaluate the potential use of “real world evidence”— ie, not from clinical trials—for approving new indications for drugs.82,83
Such evidentiary changes may increase the risk that unsafe drugs will enter the market.76,84 And most,73-75,85,86 but not all,84 studies suggest that shorter review times are deleterious.
We propose several reforms to the drug approval process: Firstly, industry funding of drug regulatory agencies should be ended; governments should fully fund agency budgets. Secondly, expedited review should be reserved for drugs likely to offer genuine clinical advances. For instance, “first in class” drugs should not automatically qualify for expedited approval since many are not superior to existing products.7 Thirdly, requirements that trials use hard clinical endpoints and active comparators should be waived only in exceptional circumstances. Fourthly, while experts who receive commercial funding may appropriately offer testimony before advisory panels evaluating drugs, such experts should not be allowed to participate in the panels’ voting or decision making.87 Finally, drugs should be required to demonstrate superiority—whether in efficacy, safety, or convenience of dosing or administration—over any existing agents to be eligible for market exclusivity.
Postmarketing surveillance
As regulatory agencies have approved more drugs based on surrogate endpoints and smaller or fewer clinical trials, they have often mandated postmarketing studies to confirm benefits or exclude serious risks.77 However, this approach has serious shortcomings. Though large postmarketing studies are critical to assuring safety (especially for rare side effects), they should not be an excuse for weakening preapproval safety requirements. And while big data approaches to pharmacosurveillance (eg, the FDA Sentinel System) hold promise, their results to date are modest and cannot substitute for clinical trials.88
Unfortunately, enforcement of mandated postmarketing studies is currently lax. The FDA has failed to fully use its authority to penalize firms that don’t complete such studies,77,78 while Health Canada has allowed firms to continue marketing drugs for years without completing required trials.79
We propose several reforms to upgrade postmarketing safety efforts. Funding for such efforts within the FDA and Health Canada should be increased to a level on par with spending for review of new drug applications, and safety offices should have equal position in these agencies’ hierarchies to offices tasked with drug approval. Safety monitoring offices should be empowered to independently order safety warnings and remove unsafe drugs from the market, and agencies should use their legal authority more aggressively to pursue drug companies that fail to complete required postmarketing studies on time. Finally, information about delays must be made publicly available.
Some of these reforms could be accomplished without legislation: since 2007, for instance, the FDA has had authority to penalize companies that failed to conduct timely postmarketing studies. Yet it has not exercised that power in any meaningful way.78 Recent legislation allows Health Canada to levy substantial fines in case of company non-compliance.89
Promotion
Drug promotion—including industry “detailing” of physicians’ offices—consumes billions of dollars annually, more than total expenditure on medical student education in the US90-92; expenditures for sales and marketing exceed those for research and development.13 In addition to diverting funds that might be better used to develop lifesaving medications, such promotion is often misleading or inaccurate.93-95 This is especially true for direct-to-consumer advertising (DTC)—now widespread in the US96 and, in attenuated form, Canada.97 Advertising that mentions the brand name of a prescription-only medicine along with its indication is banned in all other developed nations except New Zealand.
Promotional spending dwarfs the tiny budgets of the FDA and Health Canada components that regulate marketing. The FDA is overwhelmed by the sheer volume of materials to review,98,99 and Health Canada has delegated most of the regulatory oversight of promotion to third parties.97
We propose a major expansion of promotional review. Regulatory agencies need more (and more predictable) resources to carry out rigorous assessments of all promotional materials.99 They should not have to rely on funding contingent on meeting deadlines to complete reviews, which can foster a lenient approach, and money should come only from government to avoid conflicts of interest.99
Improved monitoring should be coupled with stiffer sanctions for misleading or off-label promotion. In the past, even massive fines haven’t deterred industry violations100 because, as one expert noted, “When you’re selling $1 billion a year or more of a drug, it’s very tempting for a company to just ignore the traffic ticket and keep speeding.”101 Hence, authorities should be empowered to suspend firms’ right to promote their products or, in extreme cases, pursue criminal complaints against drug executives.
While we also favor prohibiting direct-to-consumer advertising and industry detailing, constitutional challenges based on “commercial speech” rights may preclude such bans in the US.102 However, other tools are clearly constitutional, such as eliminating tax deductions for promotional activities; additionally, when alternative treatments are available, drugs promoted in these fashions might be excluded from the formulary. Industry detailing could also be countered by not-for-profit “academic detailing”103 to optimize physician prescribing practices.104
Finally, industry funding can bias continuing medical education (CME)105 and clinical guidelines.106 Licensing authorities should not accept industry funded CME for mandated credits. CME could, instead, be undertaken and coordinated by a body similar to the Australian NPS MedicineWise (www.nps.org.au), while clinical guideline development should, at a minimum, follow the recommendations outlined by the Institute of Medicine.107
Economics of a national pharmaceutical program
Although our proposal would have large economic and budgetary implications, a detailed examination of those effects is beyond the scope of this article. Others have estimated that a national pharmaceutical program for Canada could save $7.3bn of the $22bn currently spent annually on prescription drugs in that nation, although that estimate did not contemplate the new investments in drug research, development, and regulation that we advocate.108 For the US, we believe that savings on drug prices through the mechanisms detailed above could fully offset the added costs of universal, first dollar drug coverage and new public investments that we recommend.
Achieving change
Jonas Salk, inventor of the polio vaccine, eschewed patenting, declaring: “Could you patent the sun?” Today, in contrast, profiteering too often reigns, to the detriment of population health.
Our proposal calls for a fundamental reorientation of drug policy: it would make drugs more affordable for patients and society, promote innovation, strengthen efforts to assure the safety and effectiveness of medications, and upgrade the evidence available to prescribers and the public. Because drugs developed through the proposed new public pathways would remain in the public domain, they could be produced generically throughout the world, benefiting many nations.
The reforms we advocate face formidable political opposition, especially from drug firms, with those in the Fortune 500 in the US alone making total profits of $67.7bn in 2016.37 However, most Americans—both Democrats and Republicans—now favor government action to lower drug prices,109 and 91% of Canadians support a universal pharmaceutical benefit.110 These are unmistakable popular mandates for change. The trail from sentiment to policy will doubtless be arduous. Yet history is replete with examples of sweeping reforms—often enabled by unpredictable shifts in political circumstances—that overcame entrenched interests. We aim with this proposal to provide a blueprint for reform that anticipates—and may kindle—transformative changes in our nations’ pharmaceutical systems.
Contributors and sources
This proposal was drafted by a writing committee comprising Adam Gaffney (cochair), Joel Lexchin (cochair), Marcia Angell, Michael Carome, David U Himmelstein, Gordon D Schiff, Sidney M Wolfe, and Steffie Woolhandler. Other members of the US/Canadian Pharmaceutical Policy Reform Working Group were: Brook Baker, Monika Dutt, Marc-André Gagnon, Gordon Guyatt, Ritika Goel, Brian Hutchison, Richard Klasa, Michael C Klein, Danielle Martin, Barbara Mintzes, Karen S Palmer, Danyaal Raza, and Robert F Woollard.
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following interests: JL has received payments from non-profits for consulting on projects that investigated indication based prescribing and which drugs should be distributed free of charge by general practitioners. He received payment from a for-profit for being on a panel that discussed expanding drug insurance in Canada.
This proposal has been endorsed by Physicians for a National Health Program and Canadian Doctors for Medicare; authors and working group members are active in both organizations. Physicians for a National Health Program is not-for-profit organization that advocates for a single-payer healthcare system for the United States. Canadian Doctors for Medicare is a not-for-profit organization that advocates on behalf of Canada’s public single-payer system.
Provenance and peer review: Commissioned; externally peer reviewed.
1. Alpern JD, Stauffer WM, Kesselheim AS. High-cost generic drugs—implications for patients and policymakers. N Engl J Med 2014;371:1859-62. 10.1056/NEJMp1408376 25390739
2. Mailankody S, Prasad V. Five years of cancer drug approvals: innovation, efficacy, and costs. JAMA Oncol 2015;1:539-40. 10.1001/jamaoncol.2015.0373 26181265
3. Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA 2016;316:858-71. 10.1001/jama.2016.11237 27552619
4. O’Sullivan BP, Orenstein DM, Milla CE. Pricing for orphan drugs: will the market bear what society cannot? JAMA 2013;310:1343-4. 10.1001/jama.2013.278129 24084916
5. Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration approvals. JAMA Intern Med 2015;175:1992-4. 10.1001/jamainternmed.2015.5868 26502403
6. Kumar H, Fojo T, Mailankody S. An appraisal of clinically meaningful outcomes guidelines for oncology clinical trials. JAMA Oncol 2016;2:1238-40. 10.1001/jamaoncol.2016.0931 27281466
7. Lexchin J. How safe and innovative are first-in-class drugs approved by Health Canada: a cohort study. Healthc Policy 2016;12:65-75.28032825
8. Lexchin J. Those who have the gold make the evidence: how the pharmaceutical industry biases the outcomes of clinical trials of medications. Sci Eng Ethics 2012;18:247-61. 10.1007/s11948-011-9265-3 21327723
9. Topol EJ. Failing the public health—rofecoxib, Merck, and the FDA. N Engl J Med 2004;351:1707-9. 10.1056/NEJMp048286 15470193
10. Outterson K, Gopinathan U, Clift C, So AD, Morel CM, Røttingen JA. Delinking investment in antibiotic research and development from sales revenues: the challenges of transforming a promising idea into reality. PLoS Med 2016;13:e1002043. 10.1371/journal.pmed.1002043 27299990
11. Conti RM, Rosenthal MB. Pharmaceutical policy reform—balancing affordability with incentives for innovation. N Engl J Med 2016;374:703-6. 10.1056/NEJMp1515068 26933845
12. Finkelstein SN, Temin P. Reasonable Rx: solving the drug price crisis. FT Press/Pearson Education, 2008.
13. Making Medicines Affordable. A national imperative. National Academies Press, 2017.
14. Morgan SG, Gagnon M-A, Mintzes B, Lexchin J. A better prescription: advice for a national strategy on pharmaceutical policy in Canada. Healthc Policy 2016;12:18-36.27585023
15. Morgan SG, Lee A. Cost-related non-adherence to prescribed medicines among older adults: a cross-sectional analysis of a survey in 11 developed countries. BMJ Open 2017;7:e014287. 10.1136/bmjopen-2016-014287 28143838
16. Committee on Economic Social and Cultural Rights. General comment No 14. 2000. http://www.un.org…
17. Spurling GK, Mansfield PR, Montgomery BD, etal . Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med 2010;7:e1000352. 10.1371/journal.pmed.1000352 20976098
18. Collins SR, Rasmussen PW, Doty MM, Beutel S. The rise in health care coverage and affordability since health reform took effect: findings from the Commonwealth Fund Biennial Health Insurance Survey, 2014. Issue Brief (Commonw Fund) 2015;2:1-16.25807592
19 Himmelstein DU, Thorne D, Warren E, Woolhandler S. Medical bankruptcy in the United States, 2007: results of a national study. Am J Med 2009;122:741-6. 10.1016/j.amjmed.2009.04.012 19501347
20. Himmelstein DU, Woolhandler S, Sarra J, Guyatt G. Health issues and health care expenses in Canadian bankruptcies and insolvencies. Int J Health Serv 2014;44:7-23. 10.2190/HS.44.1.b 24684082
21. Congressional Budget Office. Federal subsidies for health insurance coverage for people under age 65: 2017 to 2027. 2017. https://www.cbo.gov…
22. Tamblyn R, Laprise R, Hanley JA, etal. Adverse events associated with prescription drug cost-sharing among poor and elderly persons. JAMA 2001;285:421-9. 10.1001/jama.285.4.421 11242426
23. Lohr KN, Brook RH, Kamberg CJ, etal. Use of medical care in the Rand Health Insurance Experiment. Diagnosis- and service-specific analyses in a randomized controlled trial. Med Care 1986;24(Suppl):S1-87.3093785
24. Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA 2007;298:61-9. 10.1001/jama.298.1.61 17609491
25. Sinnott SJ, Buckley C, O’Riordan D, Bradley C, Whelton H. The effect of copayments for prescriptions on adherence to prescription medicines in publicly insured populations; a systematic review and meta-analysis. PLoS One 2013;8:e64914. 10.1371/journal.pone.0064914 23724105
26 Choudhry NK, Bykov K, Shrank WH, etal. Eliminating medication copayments reduces disparities in cardiovascular care. Health Aff (Millwood) 2014;33:863-70. 10.1377/hlthaff.2013.0654 24799585
27. OECD. Health at a glance 2015: OECD indicators. 2015. 10.1787/health_glance-2015-en
28. Free prescriptions ‘saving Welsh NHS money for 10 years’. BBC News 2017 Apr 1. http://www.bbc.com…
29. Kesselheim AS, Huybrechts KF, Choudhry NK, etal. Prescription drug insurance coverage and patient health outcomes: a systematic review. Am J Public Health 2015;105:e17-30. 10.2105/AJPH.2014.302240 25521879
30. Cochrane AL. Effectiveness and efficiency: random reflections on health services. Nuffield Provincial Hospitals Trust, 1972.
31. Langret R, Migliozzi B, Gokhale K. The US pays a lot more for top drugs than other countries. Bloomberg 2015. https://www.bloomberg.com…
32. McKinnell H. A call to action: taking back healthcare for future generations. McGraw Hill, 2005.
33. Prasad V, Mailankody S. Research and development spending to bring a single cancer drug to market and revenues after approval. JAMA Intern Med 2017;177:1569-75. 10.1001/jamainternmed.2017.3601 28892524
34. Parker-Pope T, Peachman RR. EpiPen price rise sparks concern for allergy sufferers. New York Times 2016 Aug 22. https://well.blogs.nytimes.com…
35. Pollack A. Drug goes from $13.50 a tablet to $750, overnight. New York Times 2015 Sep 20. https://www.nytimes.com…
36. Experts in Chronic Myeloid Leukemia. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood 2013;121:4439-42. 10.1182/blood-2013-03-490003 23620577
37. Ranked within industries. Fortune 2017;500:f33-40.
38. Roughead EE, Lopert R, Sansom LN. Prices for innovative pharmaceutical products that provide health gain: a comparison between Australia and the United States. Value Health 2007;10:514-20. 10.1111/j.1524-4733.2007.00206.x 17970935
39. Gagnon M-A, Wolfe S. Mirror, mirror on the wall. 2015. https://www.citizen.org…
40. Kanavos P, Ferrario A, Vandoros S, Anderson GF. Higher US branded drug prices and spending compared to other countries may stem partly from quick uptake of new drugs. Health Aff (Millwood) 2013;32:753-61. 10.1377/hlthaff.2012.0920 23569056
41. Congressional Budget Office. Prices for brand-name drugs under selected federal programs. 2005. https://www.cbo.gov…
42. Two “not cost effective” drugs face being dropped from cancer drugs fund. Pharmaceutical Journal 2016. http://www.pharmaceutical-journal.com…
43. Love J. Talking drug prices. Pt 4. Drug pricing is out of control, what should be done? Plos One Blog 19 Oct 2015. http://blogs.plos.org…
44. Reichman JH. Comment: compulsory licensing of patented pharmaceutical inventions: evaluating the options. J Law Med Ethics 2009;37:247-63. 10.1111/j.1748-720X.2009.00369.x 19493070
45. Kapczynski A, Kesselheim AS. Government patent use: a legal approach to reducing drug spending. Health Aff (Millwood) 2016;35:791-7. 10.1377/hlthaff.2015.1120 27140984
46. Mundy A. Just the medicine. Wash Mon 2016. Nov/Dec. https://washingtonmonthly.com…
47. Carroll J, Winslow R. Bayer to slash by nearly half price US pays for anthrax drug. Wall Street Journal 2001 Oct 25. https://www.wsj.com…
48. Foss K. Patent war looming over drug for anthrax decision asking manufacturer to infringe necessary for Canadians’ safety, Rock says. Glove and Mail 2001 Oct 19. https://www.theglobeandmail.com…
49. Moïse P, Docteur E. Pharmaceutical pricing and reimbursement policies in sweden. OECD Health Working Papers. 2007. http://search.oecd.org…
50. Light DW, Lexchin J, Darrow JJ. Institutional corruption of pharmaceuticals and the myth of safe and effective drugs. J Law Med Ethics 2013;41:590-600. 10.1111/jlme.12068 24088149
51. Patented Medicine Prices Review Board. Annual report 2016. PMPRB, 2017.
52. Lanthier M, Miller KL, Nardinelli C, Woodcock J. An improved approach to measuring drug innovation finds steady rates of first-in-class pharmaceuticals, 1987-2011. Health Aff (Millwood) 2013;32:1433-9. 10.1377/hlthaff.2012.0541 23918488
53. Rupp T, Zuckerman D. Quality of life, overall survival, and costs of cancer drugs approved based on surrogate endpoints. JAMA Intern Med 2017;177:276-7. 10.1001/jamainternmed.2016.7761. 27898978
54. Ward DJ, Slade A, Genus T, Martino OI, Stevens AJ. How innovative are new drugs launched in the UK? A retrospective study of new drugs listed in the British National Formulary (BNF) 2001-2012. BMJ Open 2014;4:e006235. 10.1136/bmjopen-2014-006235 25344485
55. Davis C, Naci H, Gurpinar E, Poplavska E, Pinto A, Aggarwal A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13. BMJ 2017;359:j4530. 10.1136/bmj.j4530 28978555
56. Downing NS, Ross JS, Jackevicius CA, Krumholz HM. Avoidance of generic competition by Abbott Laboratories’ fenofibrate franchise. Arch Intern Med 2012;172:724-30. 10.1001/archinternmed.2012.187 22493409
57. Vokinger KN, Kesselheim AS, Avorn J, Sarpatwari A. Strategies that delay market entry of generic drugs. JAMA Intern Med 2017;177:1665-9. 10.1001/jamainternmed.2017.4650 28975217
58. Markel H. Patents, profits, and the American people—the Bayh-Dole Act of 1980. N Engl J Med 2013;369:794-6. 10.1056/NEJMp1306553 23984726
59. Correa C. Trends in drug patenting—case studies. Ediciones Corregidor 2001. http://apps.who.int/medicinedocs…
60. Sarpatwari A, Avorn J, Kesselheim AS. Factors influencing prescription drug costs in the United States—reply. JAMA 2016;316:2431-2. 10.1001/jama.2016.17299 27959994
61. Treasure CL, Kesselheim AS. How patent troll legislation can increase timely access to generic drugs. JAMA Intern Med 2016;176:729-30. 10.1001/jamainternmed.2016.1867 27183456
62. Attaran A. A modest but meaningful decision for Indian drug patents. Lancet 2014;384:477-9. 10.1016/S0140-6736(13)60845-4 24976117
63. Love J. What’s wrong with current system of funding R&D, and what are ideas for reforms? Knowledge Ecology International 2015. http://keionline.org/node/2350
64. Baker D, Chatani N. Promoting good ideas on drugs: are patents the best way? the relative efficiency of patent and public support for bio-medical research. 2002. http://cepr.net…
65. Bikdeli B, Punnanithinont N, Akram Y, etal. Two decades of cardiovascular trials with primary surrogate endpoints: 1990-2011. J Am Heart Assoc 2017;6:e005285. 10.1161/JAHA.116.005285 28325713
66. Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med 2008;358:252-60. 10.1056/NEJMsa065779 18199864
67. Glaxo agrees to pay $3 billion in fraud settlement. New York Times 2012 Jul 2. https://www.nytimes.com…
68. Hwang TJ, Carpenter D, Lauffenburger JC, Wang B, Franklin JM, Kesselheim AS. Failure of investigational drugs in late-stage clinical development and publication of trial results. JAMA Intern Med 2016;176:1826-33. 10.1001/jamainternmed.2016.6008 27723879
69. Jayadev A, Stiglitz J. Two ideas to increase innovation and reduce pharmaceutical costs and prices. Health Aff (Millwood) 2009;28:w165-8. 10.1377/hlthaff.28.1.w165 19088104
70. Mintzes B, Lexchin J, Quintano AS. Clinical trial transparency: many gains but access to evidence for new medicines remains imperfect. Br Med Bull 2015;116:43-53. 10.1093/bmb/ldv042. 26493102
71. Svensson S, Menkes DB, Lexchin J. Surrogate outcomes in clinical trials: a cautionary tale. JAMA Intern Med 2013;173:611-2. 10.1001/jamainternmed.2013.3037 23529157
72. BakerD. The benefits and savings from publicly funded clinical trials of prescription drugs. Int J Health Serv 2008;38:731-50. 10.2190/HS.38.4.i 19069290
73. Carpenter D, Zucker EJ, Avorn J. Drug-review deadlines and safety problems. N Engl J Med 2008;358:1354-61. 10.1056/NEJMsa0706341 18367738
74. Frank C, Himmelstein DU, Woolhandler S, etal. Era of faster FDA drug approval has also seen increased black-box warnings and market withdrawals. Health Aff (Millwood) 2014;33:1453-9. 10.1377/hlthaff.2014.0122 25092848
75. Olson MK. The risk we bear: the effects of review speed and industry user fees on new drug safety. J Health Econ 2008;27:175-200. 10.1016/j.jhealeco.2007.10.007 18207263
76. Lexchin J. Post-market safety warnings for drugs approved in Canada under the Notice of Compliance with conditions policy. Br J Clin Pharmacol 2015;79:847-59. 10.1111/bcp.12552 25393960
77. Moore TJ, Furberg CD. Development times, clinical testing, postmarket follow-up, and safety risks for the new drugs approved by the US Food And Drug Administration: the class of 2008. JAMA Intern Med 2014;174:90-5. 10.1001/jamainternmed.2013.11813 24166236
78. Fain K, Daubresse M, Alexander GC. The Food And Drug Administration Amendments Act and postmarketing commitments. JAMA 2013;310:202-4. 10.1001/jama.2013.7900 23839755
79. Law MR. The characteristics and fulfillment of conditional prescription drug approvals in Canada. Health Policy 2014;116:154-61. 10.1016/j.healthpol.2014.03.003 24703857
80. Kesselheim AS, Wang B, Franklin JM, Darrow JJ. Trends in utilization of FDA expedited drug development and approval programs, 1987-2014: cohort study. BMJ 2015;351:h4633. 10.1136/bmj.h4633 26400751
81. Lexchin J. Health Canada’s use of its priority review process for new drugs: a cohort study. BMJ Open 2015;5:e006816. 10.1136/bmjopen-2014-006816 25967989
82. Avorn J, Kesselheim AS. The 21st Century Cures Act—will it take us back in time? N Engl J Med 2015;372:2473-5. 10.1056/NEJMp1506964 26039522
83. Kesselheim AS, Avorn J. New “21st century cures” legislation: Speed and ease vs science. JAMA 2017;317:581-2. 10.1001/jama.2016.20640 28056124
84. Downing NS, Shah ND, Aminawung JA, etal. Postmarket safety events among novel therapeutics approved by the us food and drug administration between 2001 and 2010. JAMA 2017;317:1854-63. 10.1001/jama.2017.5150 28492899
85. Lexchin J. New drugs and safety: what happened to new active substances approved in Canada between 1995 and 2010? Arch Intern Med 2012;172:1680-1. 10.1001/archinternmed.2012.4444 23044937
86. Mostaghim SR, Gagne JJ, Kesselheim AS. Safety related label changes for new drugs after approval in the US through expedited regulatory pathways: retrospective cohort study. BMJ 2017;358:j3837. 10.1136/bmj.j3837 28882831
87. Pham-Kanter G. Revisiting financial conflicts of interest in FDA advisory committees. Milbank Q 2014;92:446-70. 10.1111/1468-0009.12073 25199895
88. Moore TJ, Furberg CD. Electronic health data for postmarket surveillance: a vision not realized. Drug Saf 2015;38:601-10. 10.1007/s40264-015-0305-9 26025018
89. Herder M, Gibson E, Graham J, Lexchin J, Mintzes B. Regulating prescription drugs for patient safety: does Bill C-17 go far enough? CMAJ 2014;186:E287-92. 10.1503/cmaj.131850 24616135
90. Frenk J, Chen L, Bhutta ZA, etal. Health professionals for a new century: transforming education to strengthen health systems in an interdependent world. Lancet 2010;376:1923-58. 10.1016/S0140-6736(10)61854-5 21112623
91. Kornfield R, Donohue J, Berndt ER, Alexander GC. Promotion of prescription drugs to consumers and providers, 2001-2010. PLoS One 2013;8:e55504. 10.1371/journal.pone.0055504 23469165
92. Gagnon M-A, Lexchin J. The cost of pushing pills: a new estimate of pharmaceutical promotion expenditures in the United States. PLoS Med 2008;5:e1. 10.1371/journal.pmed.0050001 18177202
93. Korenstein D, Keyhani S, Mendelson A, Ross JS. Adherence of pharmaceutical advertisements in medical journals to FDA guidelines and content for safe prescribing. PLoS One 2011;6:e23336. 10.1371/journal.pone.0023336 21858076
94. Othman N, Vitry A, Roughead EE. Quality of pharmaceutical advertisements in medical journals: a systematic review. PLoS One 2009;4:e6350. 10.1371/journal.pone.0006350 19623259
95. Spurling GK, Mansfield PR, Montgomery BD, etal. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med 2010;7:e1000352. 10.1371/journal.pmed.1000352 20976098
96. Greene JA, Herzberg D. Hidden in plain sight: marketing prescription drugs to consumers in the twentieth century. Am J Public Health 2010;100:793-803. 10.2105/AJPH.2009.181255 20299640
97. Lexchin J, Mintzes B. A compromise too far: a review of Canadian cases of direct-to-consumer advertising regulation. Int J Risk Saf Med 2014;26:213-25. 10.3233/jrs-140635. 25420763
98. Kiester M. DDMAC submissions. Drug Information Association, 2011. http://www.fda.gov…
99. Lexchin J. Models for financing the regulation of pharmaceutical promotion. Global Health 2012;8:24. 10.1186/1744-8603-8-24 22784944
100. Evans D. Big pharma’s crime spree. Bloomberg Markets, 2009: 72-86.
101. Wilson D. Side effects may include lawsuits. New York Times 2010 Oct 2. http://www.nytimes.com…
102. Shuchman M. Drug risks and free speech—can Congress ban consumer drug ads? N Engl J Med 2007;356:2236-9. 10.1056/NEJMp078080 17476002
103. Avorn J. Academic detailing: “marketing” the best evidence to clinicians. JAMA 2017;317:361-2. 10.1001/jama.2016.16036 28118458
104 Avorn J, Soumerai SB. Improving drug-therapy decisions through educational outreach. A randomized controlled trial of academically based “detailing.” N Engl J Med 1983;308:1457-63. 10.1056/NEJM198306163082406 6406886
105. Hager M, Russell S, Fletcher S, eds. Conference conclusions and recommendations. Continuing education in the health professions: improving healthcare through lifelong learning; 2007. http://macyfoundation.org…
106. Cosgrove L, Bursztajn HJ, Erlich DR, Wheeler EE, Shaughnessy AF. Conflicts of interest and the quality of recommendations in clinical guidelines. J Eval Clin Pract 2013;19:674-81. 10.1111/jep.12016 23731207
107. Institute of Medicine. Clinical practice guidelines we can trust. National Academies Press, 2011.
108. Morgan SG, Law M, Daw JR, Abraham L, Martin D. Estimated cost of universal public coverage of prescription drugs in Canada. CMAJ 2015;187:491-7. 10.1503/cmaj.141564 25780047
109. Kirzinger A, DiJulio B, Sugarman E, etal. Kaiser Health tracking poll—late April 2017: the future of the ACA and health care & the budget. 2017. https://www.kff.org…
110. Angus Reid Institute. Prescription drug access and affordability an issue for nearly a quarter of all Canadian households. 2017. http://angusreid.org…
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Author credentials: 1. Department of Medicine, Cambridge Hospital/Harvard Medical School, Cambridge, MA 02139, USA; 2. School of Health Policy and Management, York University, Toronto, Ontario, Canada